2.3 - Thermal Properties
Objectives:
- Understand the importance of liquid water to life on Earth.
- Know the unusual thermal properties of water - expands when freezes, large heat capacity - and their consequences.
- Know that water expands and evaporates when heated.
- Understand that warm water rises above cooler water and that this process is called convection.
Life on Earth started (probably) in the oceans. Life on Earth depends on water. Underlying these truths is the realisation that the water must of course be a liquid. Unlike a solid, a liquid consists of particles that are free to move and so water can flow and be separated into drops and ice cannot. Unlike a gas, liquids can carry dissolved elements and compounds and transport them from place to place and even through permeable materials. Without this property life could surely not exist. As an aside, it is an interesting question whether water is the only liquid that could support life. Could life have evolved elsewhere on a planet covered in other liquids? Anyway, back to water! From early experiments in Middle School all students know that water turns to ice at low temperatures and steam at high temperatures. Somewhere in between it slowly evaporates and disappears. We are all fortunate to live on a planet where the mean temperature is \(20^{\circ}\text{C}\), which is conducive to liquid water. If the Earth was closer to the Sun, the oceans would have boiled away. Further out they would have frozen solid. Sometimes, we call out location in the Solar System the "Goldilocks Zone".
There are a number of fascinating things that happen to water when we heat or cool it that have huge implications to the behaviour of the oceans.
There are a number of fascinating things that happen to water when we heat or cool it that have huge implications to the behaviour of the oceans.
- The heat capacity of water is very large, which means that it takes a large amount of energy to heat or cool water, much more than almost every other substance.
- As water is heated, evaporation occurs at the surface long before the boiling point is reached.
- As water freezes to ice, it expands and gets less dense.
- Water expands when heated.
Heat Capacity
It takes \(4200\,\text{J}\) of heat energy to raise the temperature of water by \(1^{\circ}\text{C}\). Similarly, the same amount of energy is required to cool the water down again. By comparison, it only requires \(800-950\,\text{J}\) to change the temperature of most types of rocks and sand. The atmosphere's heat capacity is highly variable and depends on humidity and pressure but is in the range of \(700-1000\,\text{J/kg C}\), remembering that \(1\,\text{kg}\) of air is much larger than \(1\,\text{kg}\) of water. The effect of this is the temperature of each cubic metre of the ocean at any given point changes much slower than that of the land, and much slower than that of the atmosphere. We can think of this as a thermal lag.
You can experience this for yourselves by visiting the beach on a hot summer's day. By mid-afternoon the sand is painfully hot but the ocean water appears cool. Come back well after dark and the beach is much cooler and by comparison the ocean feels warm. This also highlights the problem of using human senses to tell the temperature!
It takes \(4200\,\text{J}\) of heat energy to raise the temperature of water by \(1^{\circ}\text{C}\). Similarly, the same amount of energy is required to cool the water down again. By comparison, it only requires \(800-950\,\text{J}\) to change the temperature of most types of rocks and sand. The atmosphere's heat capacity is highly variable and depends on humidity and pressure but is in the range of \(700-1000\,\text{J/kg C}\), remembering that \(1\,\text{kg}\) of air is much larger than \(1\,\text{kg}\) of water. The effect of this is the temperature of each cubic metre of the ocean at any given point changes much slower than that of the land, and much slower than that of the atmosphere. We can think of this as a thermal lag.
You can experience this for yourselves by visiting the beach on a hot summer's day. By mid-afternoon the sand is painfully hot but the ocean water appears cool. Come back well after dark and the beach is much cooler and by comparison the ocean feels warm. This also highlights the problem of using human senses to tell the temperature!
LABS:
- Using two identical electric heaters, measure the temperature rise of \(1\,\text{kg}\) of sand and \(1\,\text{kg}\) of seawater after a period of time. You could use a datalogger and temperature sensor to record the data. A second experiment could involve measuring how much each sample cools after a period of time in the freezer.
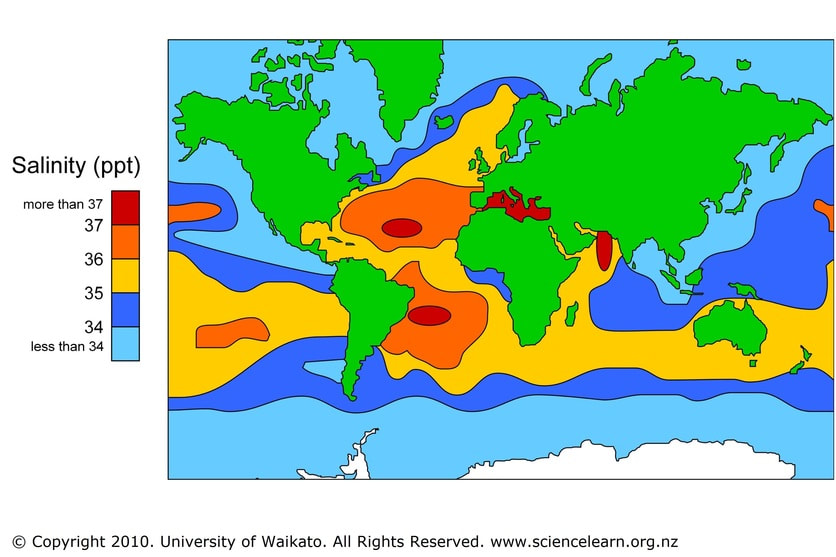
- Determine by experiment what the effect of adding salt does to the freezing and boiling points of water. What are the implications of this in colder regions such as the Baltic Sea and the polar regions?
Evaporation and Water Vapour
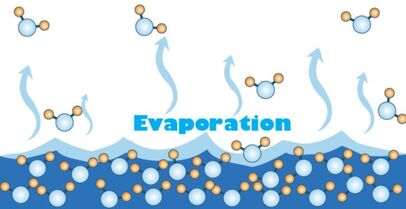
During its liquid phase water can evaporate and turn into a gas called water vapour. Evaporation tends to remove heat from the liquid or other surroundings. Evaporation occurs due to the random nature of the particles that make up a liquid. The temperature is a measure of the mean kinetic energy of the particles, but some particles will have more energy than others. If a particle is moving fast enough it can escape the cohesive forces that are binding it into the liquid. It can gain energy from heating. The primary difference between evaporation and boiling is that it occurs at the surface at any temperature. All liquids (and some solids) evaporate. A common demonstration is to wipe ethanol onto your forearm and feel how it cools your skin as it evaporates. We sweat to cool down. Air conditioners and fridges work by the evaporation of liquids inside pipes at the back.
During its liquid phase water can evaporate and turn into a gas called water vapour. Evaporation tends to remove heat from the liquid or other surroundings. Evaporation occurs due to the random nature of the particles that make up a liquid. The temperature is a measure of the mean kinetic energy of the particles, but some particles will have more energy than others. If a particle is moving fast enough it can escape the cohesive forces that are binding it into the liquid. It can gain energy from heating. The primary difference between evaporation and boiling is that it occurs at the surface at any temperature. All liquids (and some solids) evaporate. A common demonstration is to wipe ethanol onto your forearm and feel how it cools your skin as it evaporates. We sweat to cool down. Air conditioners and fridges work by the evaporation of liquids inside pipes at the back.
|
As any student who has had a damp towel knows, the rate of evaporation depends on a number of variables:
|
All of these are relevant in oceanography. The rate of evaporation is much higher in the tropics than it is in the polar regions. If the trade winds blow warm, dry air over the North Atlantic, the rate of evaporation is much higher. It is no surprise therefore that the prevailing warm southwesterly winds that affect Bermuda bring high humidity, while a cool northerly wind brings low humidity.
So, evaporation plays a part in the climate, weather and is essential for the water cycle. Without evaporation there would be no rainfall and therefore no freshwater on Earth. Evaporation changes the salinity of the water too. As seawater is warmed, water molecules escape and leaves the salt behind. A sample of seawater left in the lab will slowly become more saline as time progresses.
So, evaporation plays a part in the climate, weather and is essential for the water cycle. Without evaporation there would be no rainfall and therefore no freshwater on Earth. Evaporation changes the salinity of the water too. As seawater is warmed, water molecules escape and leaves the salt behind. A sample of seawater left in the lab will slowly become more saline as time progresses.
Thermal Expansion and Density
As water is heated it expands. This is due to the increased speed of vibration of the water molecules as they are given more and more energy. They tend to spread out more. Students do the same in the hall - allowed to move a bit they tend to spread out slightly. Given the freedom to move about they spread over the entire area of the hall. This thermal expansion is easily demonstrated in the lab. Conversely, water contracts as it cools (up to a certain point, see below). This means that the volume occupied by a fixed mass of water increases as its temperature increases. The effect of this is the density of water decreases as it warms up. This leads to convection as less dense stuff floats on more dense stuff. In a similar manner, warm air rises above cooler air. The implications of this in oceanography is that it drives the large scale ocean currents as cold dense water sinks and warm water rises. It also drives the winds of the world as warm air rise and cold air sinks and replaces it. These winds also drive the surface currents of the ocean. In effect, convection caused by thermal expansion is the major driver of the oceans, climate and weather. (see Unit 3 - Circulation and Climate)
As water is heated it expands. This is due to the increased speed of vibration of the water molecules as they are given more and more energy. They tend to spread out more. Students do the same in the hall - allowed to move a bit they tend to spread out slightly. Given the freedom to move about they spread over the entire area of the hall. This thermal expansion is easily demonstrated in the lab. Conversely, water contracts as it cools (up to a certain point, see below). This means that the volume occupied by a fixed mass of water increases as its temperature increases. The effect of this is the density of water decreases as it warms up. This leads to convection as less dense stuff floats on more dense stuff. In a similar manner, warm air rises above cooler air. The implications of this in oceanography is that it drives the large scale ocean currents as cold dense water sinks and warm water rises. It also drives the winds of the world as warm air rise and cold air sinks and replaces it. These winds also drive the surface currents of the ocean. In effect, convection caused by thermal expansion is the major driver of the oceans, climate and weather. (see Unit 3 - Circulation and Climate)
Ice Floats
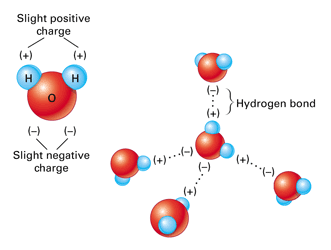
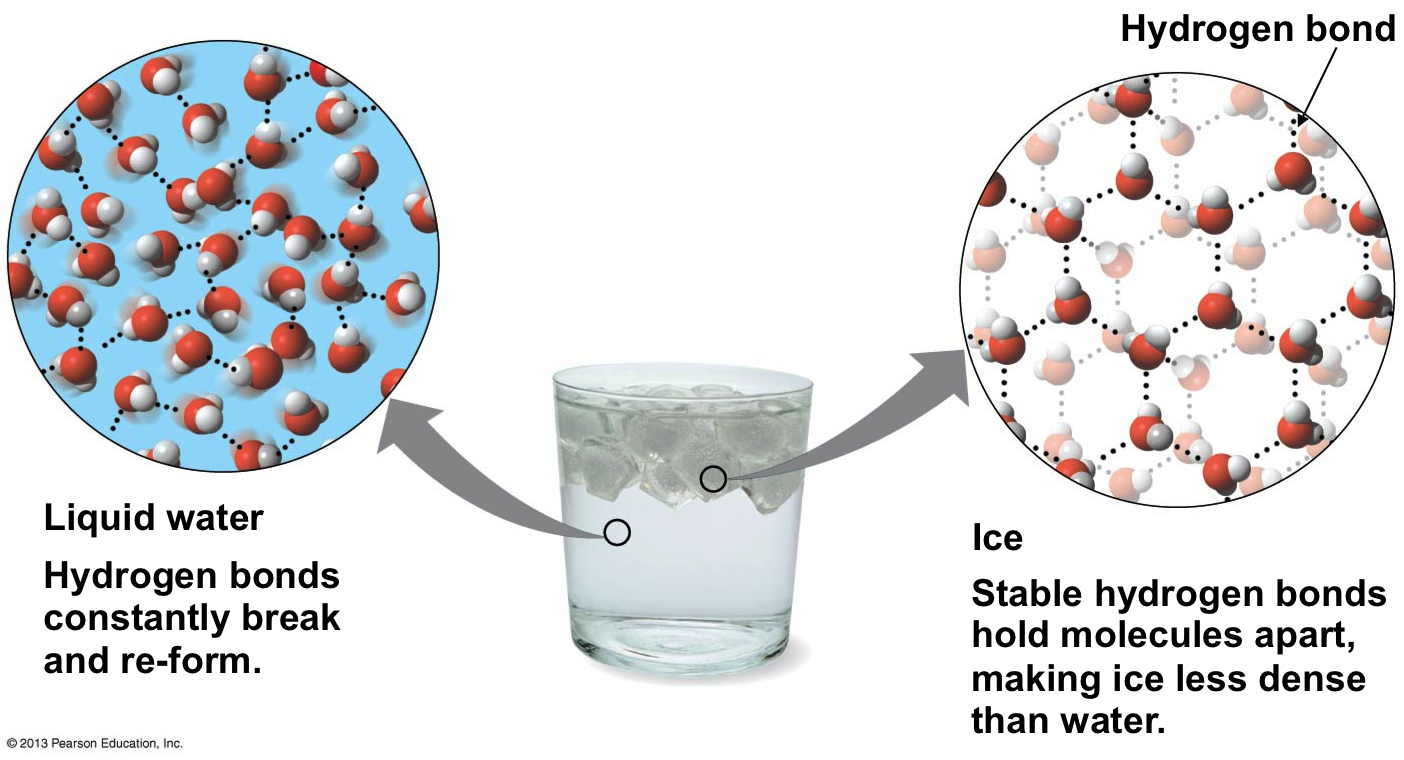
The final thermal property of water that is of great interest and importance is the unusual behaviour when it freezes into a solid form, ice. For almost every other material, the volume continues to decrease as molecular motion slows down and the material solidifies. This a major problem when casting metals - you can pour the molten metal into a mold, but if it cools too fast the metal can shrink before the excess sinks into replace it and the casting is ruined. Water on the other hand EXPANDS as it solidifies. This can be easily demonstrated using a freezer and a water bottle. As it has expanded, the mass has not changed, so it becomes less dense. The consequence of this is that ice floats on water. From experiment it is found that the maximum density of water occurs at \(4^{\circ}\text{C}\). The reason that this phenomenon happens is due to the lopsided polar nature of the water molecule itself.
The final thermal property of water that is of great interest and importance is the unusual behaviour when it freezes into a solid form, ice. For almost every other material, the volume continues to decrease as molecular motion slows down and the material solidifies. This a major problem when casting metals - you can pour the molten metal into a mold, but if it cools too fast the metal can shrink before the excess sinks into replace it and the casting is ruined. Water on the other hand EXPANDS as it solidifies. This can be easily demonstrated using a freezer and a water bottle. As it has expanded, the mass has not changed, so it becomes less dense. The consequence of this is that ice floats on water. From experiment it is found that the maximum density of water occurs at \(4^{\circ}\text{C}\). The reason that this phenomenon happens is due to the lopsided polar nature of the water molecule itself.