8.1 - Early Models of the Atom and the Discovery of the Nucleus
Objectives:
- To know the history of the discovery of the atom.
- To be able to describe the Geiger-Marsden experiment (Gold Foil) and its results.
 J. J. Thompson - discovered the electron.
J. J. Thompson - discovered the electron.
Democritus was a pre-Socratic Greek materialist philosopher (born at Abdera in Thrace ca. 460 BCE - died ca 370 BCE). Democritus was a student of Leucippus and co-originator of the belief that all matter is made up of various imperishable, indivisible elements which he called atoma (sg. atomon) or "indivisible units", from which we get the English word atom. It is virtually impossible to tell which of these ideas were unique to Democritus and which are attributable to Leucippus.
The model of the atom in the days of Newton was a tiny, hard, indestructible sphere. Although this model worked well for the kinetic theory of gases (macroscopic behaviour); new models of the atom had to be devised when later experiments revealed the electrical nature of atoms.
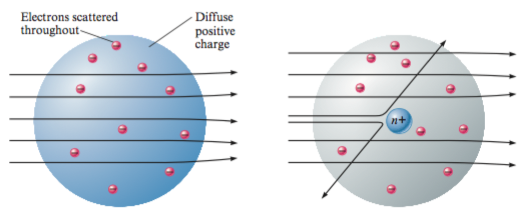
J. J. Thomson suggested a model of the atom as a volume of positive charge with negative electrons (discovered in 1895 by Thomson) embedded through the atom. This model adopted the name of ‘plum pudding’ due its resemblance of the tasty sweet (very English) dish.
Ernest Rutherford (1871 – 1937) was a physicist working at the University of Manchester. In 1911 he had two enthusiastic students called Geiger and Marsden. To keep them busy he asked them to experiment with a piece of gold foil. He suggested that they fire alpha particles at the foil and watch them bounce off the gold atoms. Alpha particles were not known to be anything else other than very heavy, very dense charged particles. He did not expect them to find anything interesting, he thought that the alpha particles would just be scattered when they passed the large ‘plum pudding’ atoms.
A steady source of alpha particles is channelled into a thin beam by a lead shield and aimed at a thin piece of gold foil. Although a piece of paper could be used to create a thin beam of alpha particles the lead shield was used to stop natural background gamma rays passing into the apparatus. As the alpha particles pass through the foil they are scattered before impacting onto a glass shield. The glass is coated on the inside with a layer of a metallic sulphide. When a particle hits the coating a small flash of light (scintillation) is produced. The light emitted can be detected using a microscope. As it was known that alpha particles were absorbed by the air in only a few cm the experiment was performed in a vacuum.
The model of the atom in the days of Newton was a tiny, hard, indestructible sphere. Although this model worked well for the kinetic theory of gases (macroscopic behaviour); new models of the atom had to be devised when later experiments revealed the electrical nature of atoms.
J. J. Thomson suggested a model of the atom as a volume of positive charge with negative electrons (discovered in 1895 by Thomson) embedded through the atom. This model adopted the name of ‘plum pudding’ due its resemblance of the tasty sweet (very English) dish.
Ernest Rutherford (1871 – 1937) was a physicist working at the University of Manchester. In 1911 he had two enthusiastic students called Geiger and Marsden. To keep them busy he asked them to experiment with a piece of gold foil. He suggested that they fire alpha particles at the foil and watch them bounce off the gold atoms. Alpha particles were not known to be anything else other than very heavy, very dense charged particles. He did not expect them to find anything interesting, he thought that the alpha particles would just be scattered when they passed the large ‘plum pudding’ atoms.
A steady source of alpha particles is channelled into a thin beam by a lead shield and aimed at a thin piece of gold foil. Although a piece of paper could be used to create a thin beam of alpha particles the lead shield was used to stop natural background gamma rays passing into the apparatus. As the alpha particles pass through the foil they are scattered before impacting onto a glass shield. The glass is coated on the inside with a layer of a metallic sulphide. When a particle hits the coating a small flash of light (scintillation) is produced. The light emitted can be detected using a microscope. As it was known that alpha particles were absorbed by the air in only a few cm the experiment was performed in a vacuum.
|
Experimental Observations
The results were startling and finished Thomson’s model of the atom. The majority of the alpha particles passed straight through the gold foil as if there was nothing there with some being scattered by small amounts. However an occasional particle was scattered through more than 90 degrees (in practice this number was less than 1 in 8000). While this was a tiny amount it was statistically a very large number of particles. Geiger and Marsden had to count many thousands of flashes before they could analyse their data. No one could believe that a massive alpha particle could bounce off a gold atom. Rutherford wrote ‘It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15 inch shell at a piece of tissue paper and it came back and hit you’. |
Rutherford was able to show that the large angle scattering was due to a single encounter between an alpha particle and an intense electric charge. He concluded that: An atom has a densely charged core (nucleus) which contains the vast majority of the mass of the atom and which is surrounded by orbiting electrons. Consequently the nucleus occupies a very small proportion of the available space. Contrary to many texts Rutherford did not know that the atom had a positive charge, at this time the charge of an alpha particle was unknown. He did use the term ‘nucleus’, but only knew that it had the same charge as an alpha particle.
This classical model of the atom as one of a dense positive nucleus surrounded by electrons in orbit is still taught in schools today and serves as an excellent foundation to build upon. Unfortunately as soon as the model was published it created problems.
Problems
There were two major issues with the planetary-style model of the atom.
Consider the electron orbits. Scientists imagined them to be similar to planets orbiting a star, with the exception that the electrons are governed by the electromagnetic force rather than the gravitational force. These electrons were undergoing circular motion and as such they must be acted upon by a centripetal force. Unfortunately it was known that if a charge (in this case an electron) is accelerated it will emit electromagnetic radiation (light). As the electron radiates (loses) energy its radius of orbit should steadily decrease and its frequency of revolution will increase. This leads to a constantly increasing amount of radiation and a collapse of the atom as the electron plunges downwards into the nucleus and everything turns into a neutron. If the theory does not match observation, then there is something wrong with the theory.
Problems
There were two major issues with the planetary-style model of the atom.
- How did the electron orbit the nucleus (atomic physics)
- How did the nucleus hold itself together against the Colomb repulsion (nuclear physics)
Consider the electron orbits. Scientists imagined them to be similar to planets orbiting a star, with the exception that the electrons are governed by the electromagnetic force rather than the gravitational force. These electrons were undergoing circular motion and as such they must be acted upon by a centripetal force. Unfortunately it was known that if a charge (in this case an electron) is accelerated it will emit electromagnetic radiation (light). As the electron radiates (loses) energy its radius of orbit should steadily decrease and its frequency of revolution will increase. This leads to a constantly increasing amount of radiation and a collapse of the atom as the electron plunges downwards into the nucleus and everything turns into a neutron. If the theory does not match observation, then there is something wrong with the theory.
ACTIVITY: linked below is the PhET Rutherford Scattering simulation by the University of Colorado. You can simulate the expected scattering of alpha particles from either the Plum Pudding or Nuclear models of the atom.
|
|
VIDEO: This is a great video of a modern version of the famous Gold Foil Experiment
|
8.2 - Spectra and Electron Orbits (Bohr Model)
Objectives:
- To know how spectral lines are seen.
- To know that that spectral lines are caused by the emission and absorption of light of specific frequencies.
- To understand that the spectral lines are due to electrons gaining or losing energy as they jump from one energy level to another.
- To be able to calculate the photon frequency from a given series of jumps.
The classical model of the atom is of an electron orbiting a nucleus. It is possible to estimate a value for the speed of an electron assuming a 2-D circular orbit for simplicity:
8.3 - The Photoelectric Effect
Objectives:
- To be able to describe the observations from the photoelectric effect and why it was so important to the story of the atom.
- To be able to explain the reason why there exists a threshold frequency and be able to interpret a graph of the observational data.
- To be able to carry out the simulation of the photoelectric effect and calculate the work function and Planck's constant from a set of data.
The need for a new theory of light
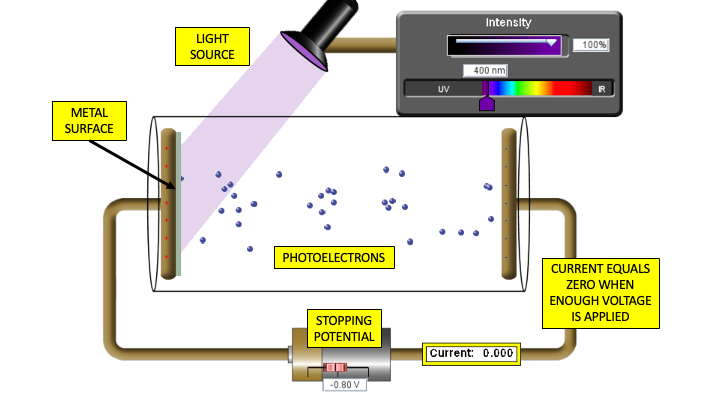
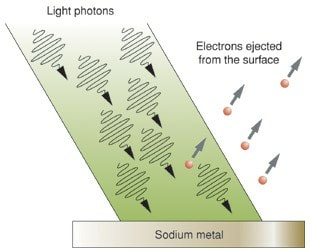
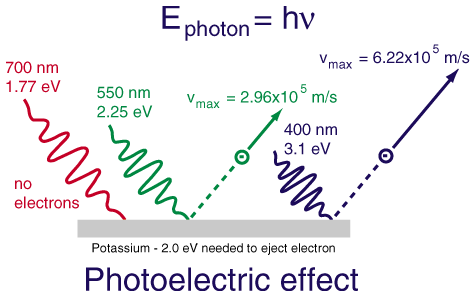
The work of Planck and then Einstein (for which he won a Noble prize in 1921) confirmed Newton's original idea that light could behave as a stream of individual particles (photons); this theory was best illustrated by the photoelectric effect. Einstein won his prize for this work and not his theories of relativity. The observation of the photoelectric effect is that when a piece of metal is illuminated by a source of electromagnetic radiation electrons are emitted from the surface. The kinetic energy of the electrons can easily be measured by applying a variable voltage and finding the voltage (stopping potential) required to make the current stop flowing.
\[KE = eV\]
The work of Planck and then Einstein (for which he won a Noble prize in 1921) confirmed Newton's original idea that light could behave as a stream of individual particles (photons); this theory was best illustrated by the photoelectric effect. Einstein won his prize for this work and not his theories of relativity. The observation of the photoelectric effect is that when a piece of metal is illuminated by a source of electromagnetic radiation electrons are emitted from the surface. The kinetic energy of the electrons can easily be measured by applying a variable voltage and finding the voltage (stopping potential) required to make the current stop flowing.
\[KE = eV\]
So far, so simple. The light energy from the lamp supplies enough energy to the electrons in the atom to allow them to leave the atom and move. However, it quickly became apparent that there were problems with this model:
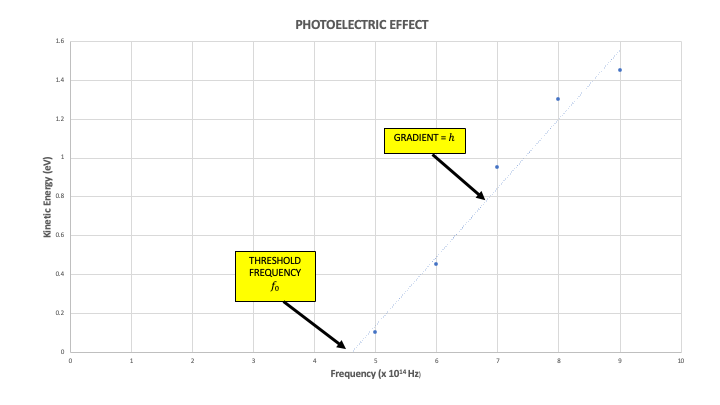
\[KE = hf - \Phi\]
- Increasing the brightness of the lamp increased the current, but not the stopping voltage. The implication is that more electrons are emitted but they all still have the same kinetic energy. As opposed to the same number of electrons that have an increased amount of kinetic energy.
- Increasing the frequency of the light (eg from red to blue) increases the kinetic energy of the electrons.
- Below a certain frequency, which is dependent on the metal, no electrons are detected - no matter how bright the light source is.
\[KE = hf - \Phi\]
Click to set custom HTML